One potential drawback of modern lightweight construction techniques is a lack of thermal massing, which refers to a building’s ability to absorb, store, and release heat energy. Without sufficient thermal mass, these types of structures can experience overheating issues in the summer and difficulty retaining warmth in the winter. As a result, mechanical heating and cooling systems are often installed to maintain indoor temperatures within a comfortable range.
However, an alternative approach involves utilizing phase change materials, or PCMs. PCMs can simulate the heat storage and release qualities provided by thermal massing. Phase change refers to the transition of a substance between solid, liquid, and gas states – a process that either absorbs or releases a significant amount of latent heat.
As the illustrations below demonstrate, PCM technology relies on latent heat storage. Unlike sensible heat storage which involves temperature changes, latent heat storage occurs without temperature variation during the phase transition process. In essence, every material could be considered a potential PCM, because under specific pressure and temperature conditions, all substances are capable of altering their physical state between solid, liquid, or gas phases.

The standard air conditioning is commonly used to keep indoor spaces cool. While effective, these systems require energy to constantly fight temperature rises. Their efficiency could improve by tapping natural day-night temperature swings through nighttime cooling strategies.
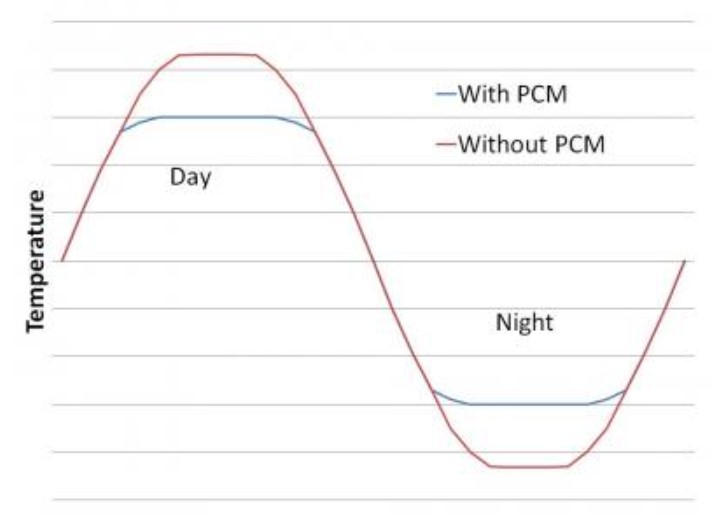
As the picture below clearly shows, PCM works to keep temperatures comfortable in a building. During a hot sunny day, it absorbs extra heat through its melting process. You can see the PCM changing from solid to liquid in the picture. Then at night, as the picture also shows, it releases that stored warmth back through resolidifying into a solid again.
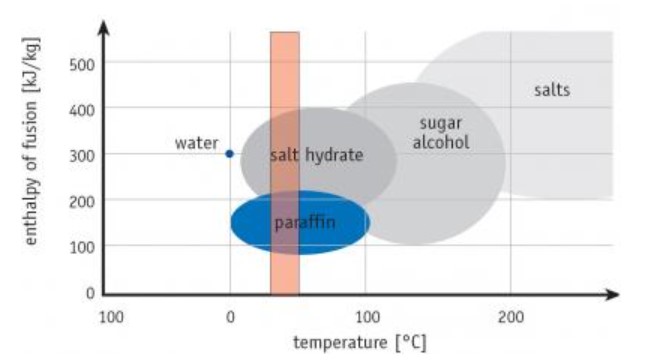
As we discussed, really any material can change phases and store heat energy that way. However not all work well or reliably for thermal storage in buildings. Figure 3 shows some PCMs and their melting ranges compared to typical home comfort levels.
You can see paraffin waxes and salt hydrates melt within the usual indoor temperature band better than others. Salts and sugar alcohols work better at higher temps needed for things like concentrating solar plants. There, salt PCM holds power from the sun for cloudy periods or night use, helping solve solar’s on-again, off-again nature.
Types of PCMs
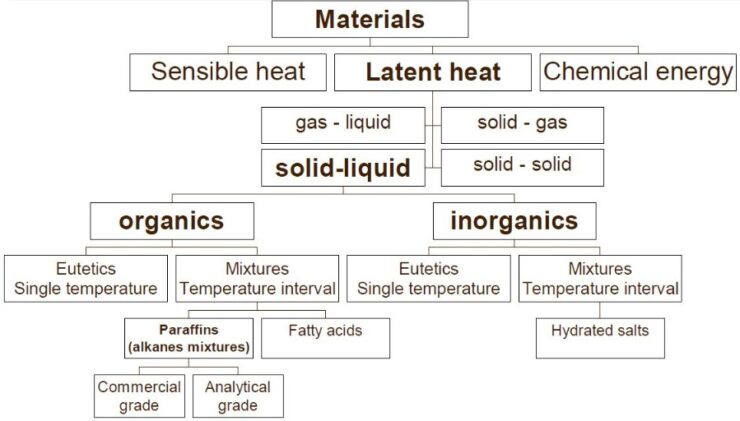
Different kinds of PCMs can be used. One main way to group them is organic or inorganic. Some common ones are:
- Paraffins (waxy organic compounds)
- Salt hydrates (inorganic materials that form when salt bonds to water)
- Fatty acids (organic compounds found in nature)
Ice can also be used as a PCM for cooling. Whether the PCM comes from organic or inorganic sources matters a lot for building construction.
As the picture below shows, PCMs can be sorted in multiple helpful ways.
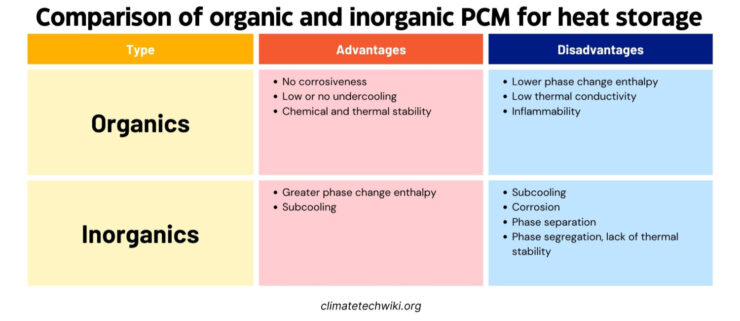
Early PCM research used inorganic salt hydrates. They have advantages like high storage capacity, won’t catch fire, are low-cost, and easy to find. But some downsides were they can rust, break down over time, resolidify unevenly, and get too cool before freezing.
It’s hard for salt hydrates to keep their big storage abilities as they melt and form new hydrates. This makes the process not work backward and their performance drops with each cycle.
Separation issues can be fixed by mixing in thickeners or gels that hold the salt together. Subcooling, when the salt starts to refreeze below its normal point, can be solved using direct heat transfer fluids or “nucleators” to trigger freezing.
Organic PCMs work better for some building uses. They are more stable chemicals, reliably melting and refreezing without slipping below freezing first like salts can. Organics also absorb well into construction materials. While costing more upfront, they equal out installed.
But organics have some potential risks also – they burn and produce fumes. A few may react with concrete or degrade over time with heat exposure. Smells and volume changes are also possible issues.
Techniques for heat transfer between PCM and the fluid cycle
Heat needs to transfer between the PCM and a heat-carrying liquid to charge and discharge the PCM, which means absorbing and releasing heat energy. Different set-ups can do this:
- Direct contact – The PCM and liquid touch directly. But they need to chemically get along long-term while the PCM freezes in small pieces, allowing heat flow during melting.
- Macro-capsules – Plastic shells around the PCM are common. They’re usually a few centimeters wide and made of a neutral plastic that doesn’t react to the PCM or liquid.
- Micro-encapsulation – A newer method coating individual PCM particles in a polymer skin just micrometers thick. This creates a huge surface area for heat exchange.
Advantages and disadvantages of PCM use compared to conventional water storage
PCM energy storage has some advantages over just using water:
- It can hold more heat per volume than sensibly heating water alone. This means smaller storage tanks. As long as small temp differences work.
- PCM stays about the same temp when soaking up and releasing heat.
- Backup generators and their pollution emissions may be needed less often.
But PCM also has downsides compared to water storage:
- It costs more upfront.
- Power output is limited while the PCM is solid since heat moves through slower. This affects the max size.
- There’s less experience with PCM lasting through thousands of charge-discharge cycles long-term.
- Risk that the PCM solution breaks down or the casing keeping it in breaks over time.
Contribution to economic development
Using materials that change from solid to liquid at certain temperatures can help our economy in some important ways. When these phase change materials (PCMs) are used to store heat or cold, it reduces how much power we need to make at peak times.
That’s important because during really hot or cold weather, a lot more electricity is used all at once. Building new power plants or backup generators to meet these high demands costs a lot of money. Using PCMs means we wouldn’t need to do as much of that.
For example, in California the population is growing fast especially in hot southern areas. During heat waves, everyone uses air conditioning at the same time. This puts a big strain on the electric grid. PCMs in buildings could save cold air until it’s needed, taking pressure off power plants during peak hours.
PCMs are also helpful for storing energy made at times when not many people are using power. Renewable sources like wind farms often make the most energy at night when winds are strong. But demand is low then since most people are asleep! Batteries or PCMs can save that electricity until the daytime when it’s needed.








